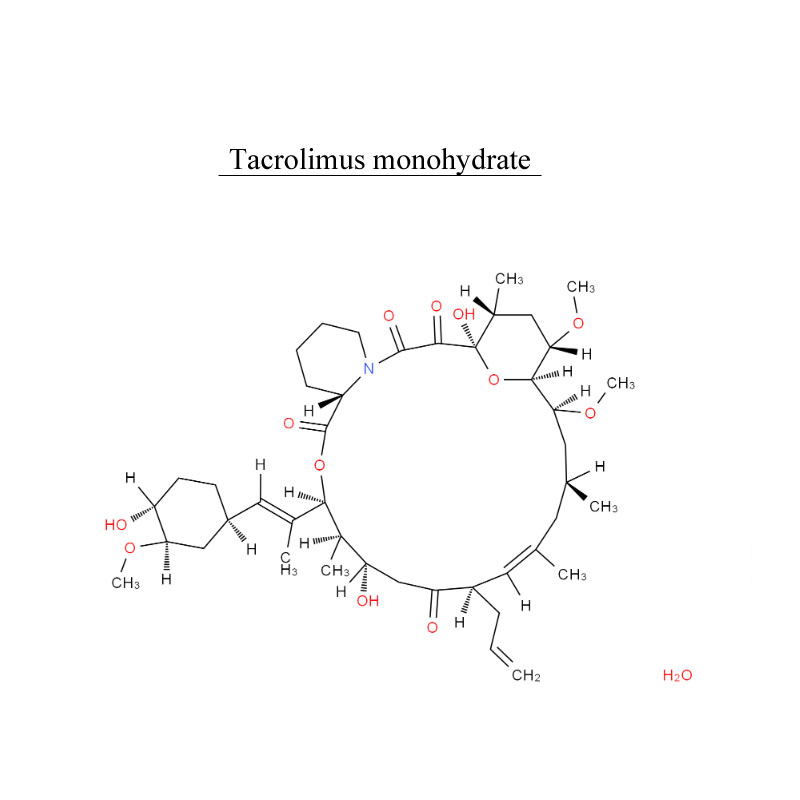
 Payment: T/T, L/C Product Origin: China Shipping Port: Beijing/Shanghai/Hangzhou Production capacity: 1kg/month Order(MOQ): 1g Lead Time: 3 Working Days Storage condition: Stored in cool, dry place, room temperature. Package material: vial, bottle Package size: 1g/vial, 5/vial, 10g/vial, 50g/bottle, 500g/bottle Safety information: UN 2811 6.1/PG 3
Payment: T/T, L/C Product Origin: China Shipping Port: Beijing/Shanghai/Hangzhou Production capacity: 1kg/month Order(MOQ): 1g Lead Time: 3 Working Days Storage condition: Stored in cool, dry place, room temperature. Package material: vial, bottle Package size: 1g/vial, 5/vial, 10g/vial, 50g/bottle, 500g/bottle Safety information: UN 2811 6.1/PG 3| Item | Specification |
| Appearance | White or almost white crystalline powder |
| Identification | IR, HPLC |
| Solubility | Very soluble in methanol, freely soluble in N,N dimethylformamide and in alcohol, practically in soluble in water. |
| Residue on ignition | ≤0.10 % |
| Organic impurities (procedure-2) | Ascomycin 19-epimer ≤0.10 % |
| Ascomycin ≤0.50 % | |
| Desmethyl tacrolimus ≤0.10 % | |
| Tacrolimus 8-epimer ≤0.15 % | |
| Tacrolimus 8-propyl analog ≤0.15 % | |
| Unknown impurity -I ≤0.10 % | |
| Unknown impurity -II ≤0.10 % | |
| Unknown impurity -III ≤0.10 % | |
| Total impurities ≤1.00 % | |
| Optical rotation (on as is basis) (10mg/ml in N,Ndimethylformamide) | -110.0° ~ -115.0° |
| Water content (by KF) | ≤4.0% |
| Residual solvents (by GC) | Acetone ≤1000ppm (In-house) |
| Di-isopropyl ether ≤100ppm (In-house) | |
| Ethyl ether ≤5000ppm | |
| Acetonitrile ≤410ppm | |
| Toluene ≤890ppm | |
| Hexane ≤290ppm | |
| Microbial test (in house) | Total aerobic microbial count ≤100cfu/gm |
| Total yeast and mould count ≤10cfu/gm | |
| Specified organisms (Pathogens) (E.coil, salmonella sps., S.aureus. Pseudomonas aeruginosa) should absent | |
| Assay (by HPLC) (on anhydrous and solvent free basis) | 98%~102% |