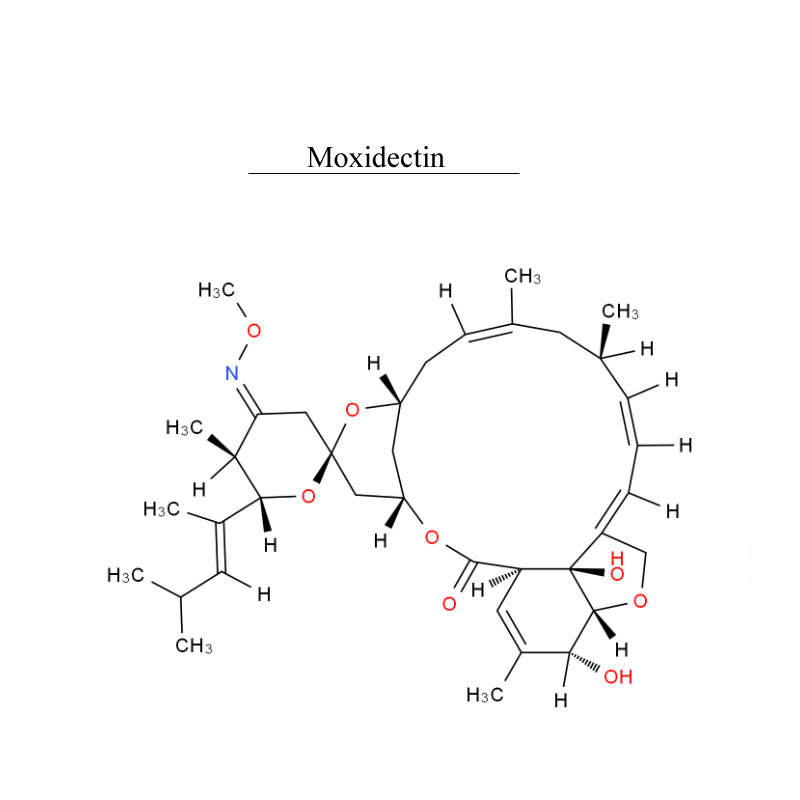
 Payment: T/T, L/C Product Origin: China Shipping Port: Beijing/Shanghai/Hangzhou Order (MOQ): 25kg Lead Time: 3 working days Production capacity: 100kg/month Storage condition: Stored in cool, dry place, room temperature. Package material: drum Package size: 25kg/drum Safety information: UN2811 6.1/PG 3
Payment: T/T, L/C Product Origin: China Shipping Port: Beijing/Shanghai/Hangzhou Order (MOQ): 25kg Lead Time: 3 working days Production capacity: 100kg/month Storage condition: Stored in cool, dry place, room temperature. Package material: drum Package size: 25kg/drum Safety information: UN2811 6.1/PG 3| Item | Specification |
| Appearance | White or pale yellow amorphous powder |
| Identification | IR Spectrum of sample corresponds to that of reference substance |
| The retention time of the major peak of the sample solution corresponds to that of the standard solution, as obtained in the assay | |
| Water | ≤1.3% |
| Residue on ignition | ≤0.2% |
| Heavy metals | ≤20ppm |
| Assay | 92.0-102.0% (anhydrous Substance) |
| Organic Impurities | |
| Early-eluting impurities | Moxidectin butenyl analog ≤1.5% |
| 5’-Demethyl Moxidectin ≤0.5% | |
| Moxidectin Pentenyl Analog ≤1.5% | |
| Moxidectin 17a-epimer ≤2.5% | |
| Sum of moxidectin 19-s-17a-ene and moxidectin ethyl isomers (E+F) ≤1.7% | |
| Mibemycin B analog (moxidectin open ring) ≤1.5% | |
| Any other individual impurity eluting before milbemycin B analog (moxidectin open ring) ≤0.5% | |
| Late Elutomg impurities | Moxidectin deoxydienea, and 4’-Methylthiomethoxymoxidectin (H+I) ≤ 1.0% |
| 20b-Methylthiomoxidctin (J) ≤ 0.5% | |
| 20-Nitrobenzoylmoxidectin (K) ≤ 0.5% | |
| Any other individual impurity eluting after the milbemycin B analog (Moxidectin open ring) ≤ 0.5% | |
| Total organic Impurities | ≤7.0% |
| Solvent residue | Methanol ≤ 3000ppm Methylene Chloride ≤ 300ppm Isopropyl Acetate ≤ 5000ppm N-Heptane ≤ 5000ppm |
| BHT | 0.3%-0.6% |