 Payment: T/T, L/C Product Origin: China Shipping Port: Beijing/Shanghai/Hangzhou Production capacity: 100kg/month Order(MOQ): 25kg Lead Time: 3 Working Days Storage condition: Stored in cool, dry place, room temperature Package material:drum Package size:25kg/drum Safety information: Not dangerous goods
Payment: T/T, L/C Product Origin: China Shipping Port: Beijing/Shanghai/Hangzhou Production capacity: 100kg/month Order(MOQ): 25kg Lead Time: 3 Working Days Storage condition: Stored in cool, dry place, room temperature Package material:drum Package size:25kg/drum Safety information: Not dangerous goods| Item | Specification |
| Appearance | White solid |
| Identification | IR |
| Identification of chlorides | Comparable to stand |
| Melting point | 211℃-215℃ |
| Total impuities | ≤ 0.1% |
| Individual impuity | ≤0.05% |
| Loss on drying | ≤0.05%2hrs at 105℃ |
| Sulpated ash | 0.05% max |
| Ph of standard solution | 5-6 |
| Solution(1% soltion in water) | colorless |
| Total Heavy metals (As Pb Cd Cr Ni) | ≤20ppm |
| Residual solvents | Acetone<5000ppm |
| Methylbenzene<890ppm | |
| Thf<720ppm | |
| Methanol<3000ppm | |
| Dichloromethane<600ppm | |
| Ethyl acetate<5000ppm | |
| N-hexane<290ppm | |
| Ethyl alcohol<5000ppm | |
| Purity (HPLC) | ≥99% |
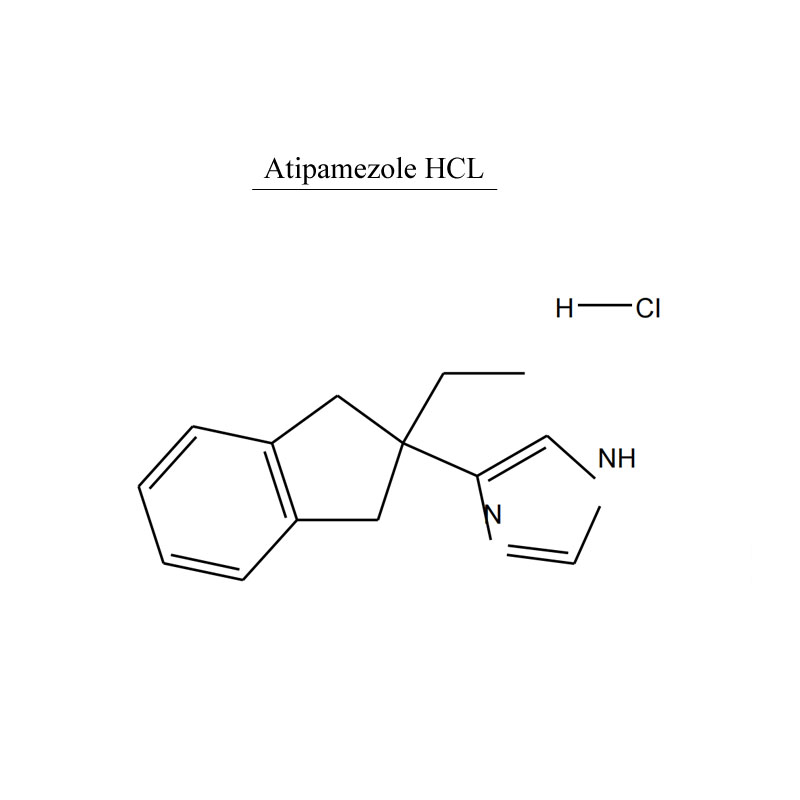
Atipamezole hydrochloride, chemical name: 4-(2-ethyl-2-indan) imidazole hydrochloride. Atipamezole hydrochloride competitively inhibits alpha 2 adrenergic receptors, and is an effective α2 adrenergic receptor blocker, which selectively and competitively inhibits α2 adrenergic receptors.
Its pharmacological effects are to reduce sedation, lower blood pressure, increase heartbeat and respiratory rate, and reduce analgesic effects of α2-adrenoceptor blockers, which are mainly used in the recovery of anesthesia after surgery on animals.
| Item | Specification |
| Appearance | Yellow to orange powderodorless. |
| Identification | IR, HPLC |
| Residue on ignition | ≤0.5% |
| Related substances | |
| At 303nm | Amphotericin A ≤2.0% |
| Individual unknown impurity ≤1.0% | |
| At 383nm | Amphotericin X1 ≤4.0% |
| Individual unknown impurity ≤2.0% | |
| Total impurities | ≤15.0% |
| Residual solvents | Acetone ≤0.5% |
| Methanol ≤0.3% | |
| Microbiological limit | Aerobic microbial count ≤1000cfu/g |
| Molds & yeasts ≤100cfu/g | |
| Escherichia coli Absent in 1g | |
| Bacterial endotoxins | <1.0EU/mg |
| Assay | ≥850 amphotericin B units/mg, calculated on the dried basis |